
Migraine in Adults Toolkit
Sponsored by Amgen
FOR PRIMARY CARE AND OTHER GENERAL PRACTITIONERS
FOR PRIMARY CARE AND OTHER GENERAL PRACTITIONERS
Clinical Phases of Migraine

Migraine has a complex pathophysiology that is becoming better understood.2 Recent studies have shown that several anatomic regions and molecular pathways underlie the multifaceted symptoms across all phases of migraine.13-16
ICHD-3 Diagnostic Criteria for Migraine
- Headache attack duration
- Headache attack lasts 4–72 hours (when untreated or unsuccessfully treated)
- Headache characteristics
- Headache has ≥2 of the following 4 characteristics:
- Unilateral location
- Pulsating quality
- Moderate or severe pain intensity
- Aggravation by or causing avoidance of routine physical activity (eg, walking or climbing stairs)
- Headache has ≥2 of the following 4 characteristics:
- Non-headache symptoms
- During headache, the patient has ≥ 1 of the following:
- Nausea and/or vomiting
- Photophobia and phonophobia
- During headache, the patient has ≥ 1 of the following:
- Not better accounted for by another ICHD-3 diagnosis
Migraine diagnosis is described in the third edition of the ICHD (ICHD-3), developed by the IHS20
Distinguishing from Other Headaches

Considerations for the Differential Diagnosis of Migraine
An accurate diagnosis of migraine depends on obtaining an accurate patient history.101 However, because of overlapping symptoms, differentiating migraine from other headache disorders can be challenging.18,22-25
Characteristic symptoms of migraine facilitate differential diagnosis from other primary headache disorders, including tension-type and cluster headache.20
Key distinctive features of migraine are:20,26-28
- Unilateral location
- Long duration (4–72 hours)
- Frequency
- Associated symptoms such as nausea and/or vomiting
- Sensitivity to light and sound or to touch
While these are distinctive features, migraine can also be bilateral and may not be associated with nausea.28,29
Medication Overuse Headache
- Medication overuse headache (MOH) is a type of secondary headache disorder caused by overuse of certain acute medications used for treatment of migraine attacks.
- Both migraine-specific acute medications (eg, triptans, ergotamines, and ditans)34,46 and non-migraine-specific acute medications (eg, analgesics and nonsteroidal anti-inflammatory drugs)45 are approved by regulatory agencies and/or recommended by professional society guidelines for relief from migraine attacks.
- Although some acute medications are indicated and/or recommended for aborting attacks, acute treatments can be overused, potentially leading to medication overuse headache (MOH), a type of secondary headache disorder.20
- MOH is more common with narcotics and barbiturates, which in addition to their habit-forming potential, is the reason that AHS guidelines do not recommend their regular use for migraine treatment.34,41,47
- Approximately 50% of patients with chronic migraine have MOH that may revert to episodic headache after drug withdrawal.20 As such, it is recommended that acute treatments be limited to an average of 2 headache days34 per week, and preventive treatment is considered for patients observed exceeding this limit.45 Also, it is advised that acute treatments not be used in anticipation of a migraine attack.48
ICHD-3 Criteria for MOH
- Headache occurring on ≥ 15 days per month in a patient with a preexisting headache disorder
- Regular overuse for > 3 months of ≥ 1 drug that can be taken for acute and/or symptomatic treatment of headache, with medication overuse is defined as:
- ≥ 10 days per month for ergot derivatives, triptans, opioids, combination analgesics,* and a combination of drugs from different classes that are not individually overused
- ≥ 15 days/month for nonopioid analgesics, acetaminophen, and NSAIDs
- Not better accounted for by another diagnosis
*Drugs of ≥ 2 classes, each with an analgesic effect or acting as adjuvants.
FOR NEUROLOGISTS AND OTHER SPECIALISTS
FOR NEUROLOGISTS AND OTHER SPECIALISTS
Migraine Neuroimaging Techniques
Clinical studies have used neuroimaging techniques to assess the structural and functional effects of migraine.49-51
- Used to visualize anatomic properties of the brain, including:49
- Size and volume of brain structures49
- Thickness of cortical areas (e.g. gray matter)49,52
- White matter tracts52,53
- Used to detect brain damage and abnormalities49
- Includes magnetic resonance imaging (MRI), computed tomography (CT), and diffusion tensor imaging (DTI)49
- Used to define regions that are active during specific behaviors and cognitive activities49,54
- Can include the study of stimuli-induced responses or “at rest”54,55
- Indirect measure of neural activity in the brain:
- Functional magnetic resonance imaging (fMRI)–blood oxygen level–dependent (BOLD) signal49,54
- Positron emission tomography (PET) – metabolism associated with performing a task/behavior49,54
Role of CGRP Signaling in Migraine Pathophysiology
Calcitonin Gene-Related Peptide (CGRP)
CGRP is a 37–amino acid neuropeptide.59 It is a member of the calcitonin family of peptides that also includes amylin (AMY), adrenomedullin (AM), and calcitonin.61,62
Clinical evidence suggests that CGRP may play a causal role in migraine.63-65
Plasma CGRP levels (measured from external jugular blood) have been shown to increase significantly during migraine attacks63 and return to normal following relief of migraine pain with triptan therapy.64 Infusion of CGRP can induce migraine-like attacks in individuals who suffer from migraine.65

Infusion of CGRP can induce migraine-like attacks in individuals who have migraine.48

CGRP Receptor (CGRP-R)
The calcitonin family of peptides binds transmembrane-bound, G-protein receptors that share subunits in different configurations. These are referred to as the calcitonin family of receptors. The CGRP receptor (CGRP-R) is a member of the calcitonin family of receptors.60
Each receptor is a heterodimer consisting of a receptor activity-modifying protein (RAMP) and either the calcitonin receptor (CTR) or calcitonin receptor-like receptor (CLR) G-protein–coupled receptor (GPCR) signaling subunit.60
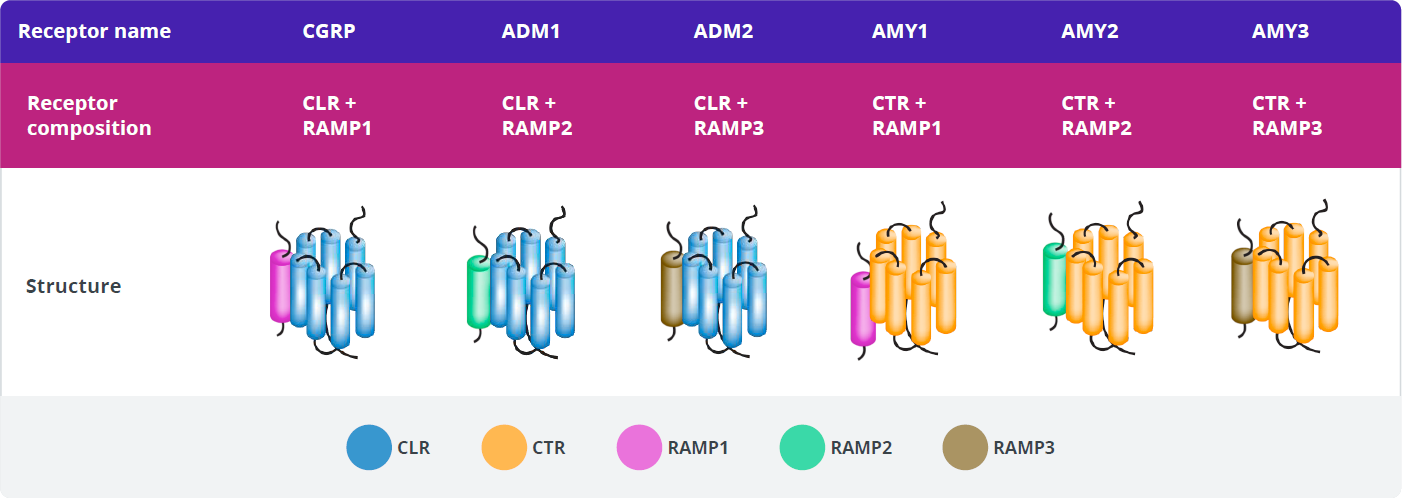
There are three distinct types of RAMPs, designated RAMP1, RAMP2, and RAMP3, each encoded by a separate gene.
Creation of a functional CGRP-R requires the co-expression of both CLR and RAMP1 and the formation of a heterodimer, which translocates from the endoplasmic reticulum to the cell membrane.66-69

Activation of G-protein–coupled receptors involves a cyclical model, whereby receptors can be recycled back to the cell surface.70,71 Ligand binding leads to transient receptor activation and induces a cellular response via second-messenger signaling.70,71 Receptors are then inactivated and internalized via endocytosis before being trafficked to either recycling or degradative pathways.66,70-72
CGRP and CGRP-R Localization
CGRP-Rs are found in multiple areas involved in migraine pathophysiology, including:73-75
- Trigeminal ganglion
- Cerebral and meningeal vasculature
- Brainstem (eg, trigeminal nucleus caudalis)
- Brain (eg, thalamus)
CGRP-Rs are also expressed on numerous cell types, such as:68,73-76
- Vascular smooth muscle cells
- Neurons
- Glial cells
- Mast cells
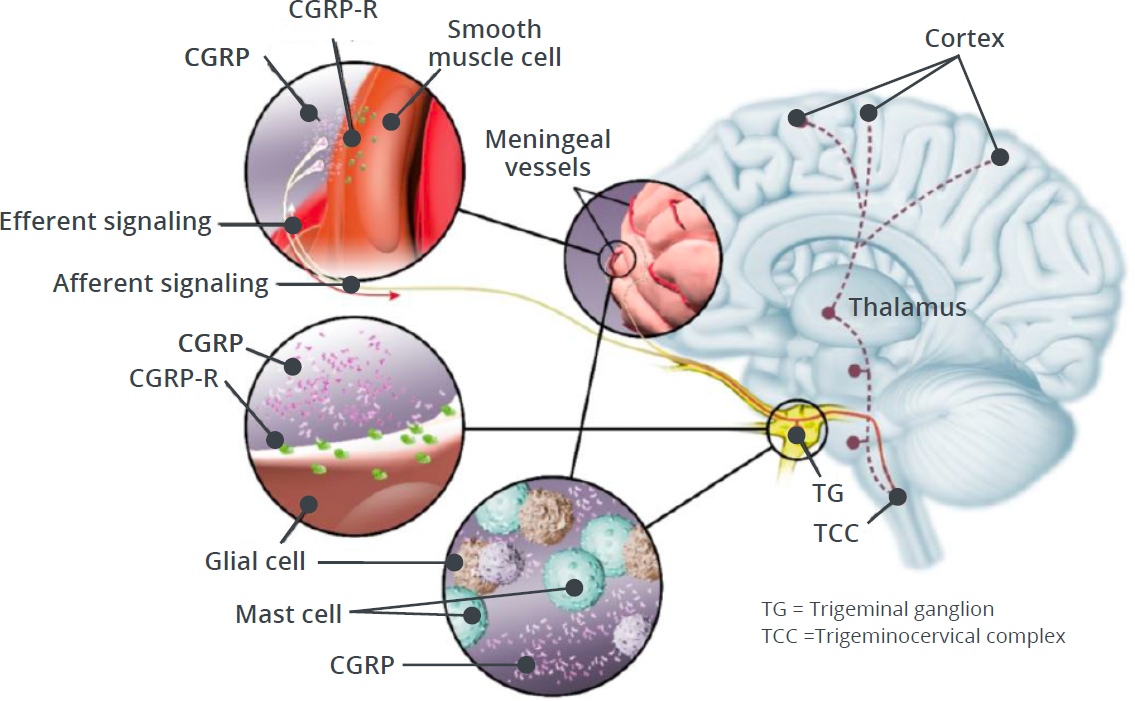
CGRP and CGRP-R Signaling
Activation of CGRP-R in the trigeminovascular system plays a critical role in peripheral and central events that ultimately lead to the experience of migraine pain.73,15,77
Following nerve stimulation, CGRP is released from its storage vesicles through the process of calcium-dependent exocytosis.59
This peripheral release of CGRP from trigeminal nerve endings is thought to trigger multiple responses induced by CGRP-R binding, which eventually lead to the sensitization of nociceptor trigeminal neurons.73,15
The stimulation of peripheral nociceptive trigeminal neurons is hypothesized to relay the migraine pain signal through the brainstem into the brain, ultimately leading to the experience of migraine pain.77,12
Central effects of CGRP may involve pain transmission through sensitization and activation of central processes (eg, feedback from a sensitized brain).77
The complex role of CGRP–CGRP-R signaling in migraine pathophysiology may involve multiple processes in both the central and peripheral nervous systems, including:
- Vasodilation1,2,6,9,10
- Nociceptor activation (peripheral)1,2,6,9,10
- Neurogenic inflammation1,2,6,9,10
- Cortical-spreading depression1,6,10
- Central trigeminal sensory activation2,6,9
- Hypothalamic dysfunction and descending control of brainstem structures6,11
- Central sensitization6,9
Research has yet to determine which of these processes play a causal role in, or if they occur as a result of, or in parallel with, migraine. Additional processes yet to be defined may also be involved.73,15
CGRP-R and AMY1-R Expression
In addition to binding to CGRP-R, CGRP has high affinity for another calcitonin receptor, the amylin 1 receptor (AMY1-R).60,61 These 2 receptors have the RAMP1 subunit in common.60
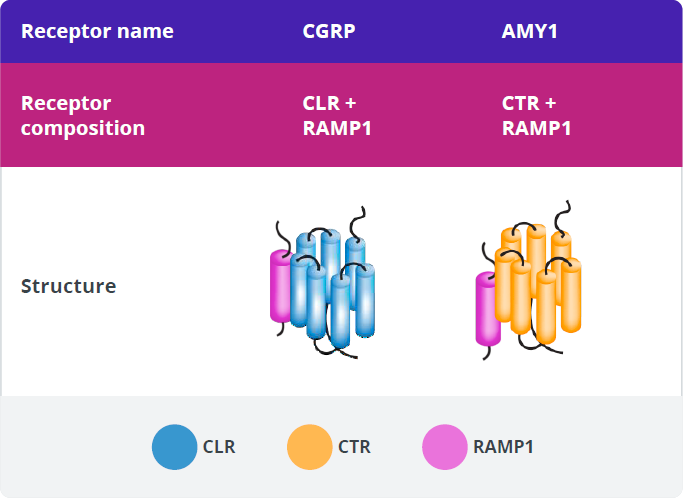
CGRP, amylin (AMY), and their receptors are expressed in various tissues and play established roles in some key physiologic processes.59,60,66,76,86-96,81-85 CGRP signaling through CGRP-R plays several firmly established physiologic roles, including nociception, sensory modulation, and vasodilation.59
The main source of amylin ligand is the pancreatic β-cells, and AMY1-R is expressed in the vasculature and the trigeminal ganglion.61,81-83
Various roles for AMY1-R have been suggested as a result of its location and the expression pattern of its ligands.62,81,82 Amylin signaling through AMY1-R plays well-established physiologic roles in regulating postprandial glucose levels, glycemic control, and satiation.81,82

Of the calcitonin receptors, only the CGRP receptor has been implicated in migraine pathophysiology.60 The roles of the other receptors in migraine pathophysiology are currently unknown.59,60
Importance of Individualized Treatment Plans and Setting Expectations
Despite guideline recommendations of a formal, individualized management plan,99 many patients report not receiving appropriate treatment and follow-up care.30,36
Because the severity, frequency, and characteristics of migraine vary among patients and, often, within patients over time, and symptom profiles or biomarkers that predict efficacy and potential side effects for patients have not yet been identified, optimizing treatment for particular patients remains challenging.99
At present, treatment plans are individualized based on the following:99
- Patient preference
- Status of pregnancy, lactation, or plans to conceive
- Frequency and severity of attacks
- Presence, type, and severity of associated symptoms
- Attack-related disability
- Prior treatment response
- Presence of comorbid and coexistent illness
- Contraindications (eg, cardiovascular disease)
- Factors such as body habitus and physiologic measures (eg, blood pressure, heart rate)
- Use of concomitant medications
A period of trial with different treatments may be necessary before treatment can be optimized.99 Additionally, it is important to establish realistic expectations with patients when discussing migraine treatment.99
- 50% reduction in the frequency of days with headache or migraine
- Significant decrease in attack duration, as defined by the patient
- Significant decrease in attack severity, as defined by the patient
- Improved response to acute treatment
- Reduction in migraine-related disability and improvements in functioning in important areas of life
- Improvements in health-related quality of life (HRQoL) and reduction in psychological distress because of migraine
Migraine may have potential long-term clinical and pathophysiological implications for patients if not managed appropriately.1,107
Assessing Treatment Effectiveness
After initiating a new pharmacologic treatment for migraine or changing an existing treatment, regular follow-up visits are recommended to assess changes in the frequency and severity of attacks, and to evaluate migraine-related symptoms.2 Follow-up visits are also recommended to identify and address AEs associated with the treatment, monitor medication use, and ensure adherence to therapy.2
To accurately evaluate treatment effectiveness, AHS guidelines recommend that patients be assessed after 3 months (after starting monthly treatments), or 6 months (after starting quarterly treatments).99
For a more accurate assessment of treatment effectiveness, it is recommended that patients keep a headache diary to capture changes in attack frequency and severity as well as medication use.2,99 Treatment effectiveness can also be evaluated using a number of available validated patient-reported outcome (PRO) tools.99 Assessing a patient’s symptoms prior to treatment and routinely throughout treatment can help determine change in duration and severity of symptoms, functional disability, and quality of life (QoL) following therapy.3
After treatment is initiated:
- Encourage the patient to keep a headache diary to assess treatment efficacy*,2,99
- Assess the number and frequency of headache days after treatment initiation2
- Assess the number of days for which acute medication was needed
- Use PRO tools to measure duration and severity of symptoms, and assess the impact of treatment on functional disability and improvement in QoL99
*A headache diary should be started as soon as treatment is initiated.
MULTIPLE AUDIENCES
MULTIPLE AUDIENCES
Treatment Overview
Migraine can be treated with acute and preventive treatment.97,98 These two strategies for treating migraine have distinct but complementary treatment goals.98,99
Acute Treatment — Used to abort a migraine attack97-99
| Potential benefits | Limitations |
|---|---|
| May provide symptom relief97-99 | Adverse events can include tolerability issues97-99 |
| Risk of medication overuse headache (MOH)99 |
Preventive Treatment — Used to reduce the frequency, duration, and/or severity of attacks99
| Potential benefits | Limitations |
|---|---|
| May provide symptom relief97-99 | Often associated with issues of patient adherence99 |
| May reduce overall cost associated with migraine treatment99 | Side effects can include depression, cognitive dysfunction, somnolence, constipation, and weight gain100,101 |
Treatment classes for acute and/or preventive migraine therapy
Patients with migraine who have frequent and/or severe attacks may require both acute and preventive treatment approaches.97,98
A number of drug classes used in the acute and/or preventive management of migraine:99
| Acute | Preventive |
|---|---|
| Triptans98,99 | Anticonvulsant drugs104 |
| Ergotamine derivatives98,99 | Beta -blockers99,104 |
| Ditans102 | Neuromodulation devices99 |
| Gepants103 | Neurotoxins99 |
| Neuromodulation devices99 | Antidepressants99,104 |
| Nonsteroidal and anti-inflammatory drugs (NSAIDs)98,99 | Angiotensin-converting enzyme (ACE) inhibitors99,104 |
| Analgesics98 | Angiotencin receptor blockers99,104 |
| Opioids98,99 | Alpha-agonists99,104 |
| Antihistimines99 | |
| Triptans99,104 | |
| CGRP monoclonal antibodies (mAbs)99 |
AHS Treatment Recommendations
AHS Recommendations for Acute Treatment
According to AHS consensus statement, all patients with migraine should be offered a trial of acute treatment.99
- Goals of acute treatment include achieving rapid freedom from pain and associated symptoms and restored ability to function.99
- Considerations for acute treatment include those related to efficacy, safety, tolerability, comorbidities, and concomitant medications.97,99
- Migraine-specific and general pain medications are recommended for the acute treatment of migraine.97-99 Specifically, the AHS recommends the use of NSAIDs, nonopioid analgesics, or combination therapy for mild-to-moderate attacks and migraine-specific agents for moderate or severe attacks and mild-to-moderate attacks that respond poorly to NSAIDs or combination therapy.99
Regardless of which acute treatment is prescribed, patients should be instructed to treat at the first sign of pain to improve the probability of achieving freedom from pain and reduce attack-related disability.99
AHS-listed drugs for acute treatment of migraine99
General pain medications
- NSAIDs
- Combination therapy
Acute migraine-specific medications
- Triptans
- Ergotamine derivatives
Emerging acute treatments
- Gepants
- Ditans
AHS Recommendations for Preventive Treatment
Preventive treatments are an important part of the overall approach for a proportion of patients with migraine, and multiple evidence-based guidelines are available.99 However, epidemiology data support that preventive treatment is underutilized, as only about one-third of patients with migraine who qualify for preventive treatment receive it.31,105
Preventive treatment is recommended for patients with elevated headache frequency, increased symptom severity, and/or impaired functioning.99
Identifying Patients for Preventive Treatment
Patients are often selected for preventive treatment based on attack frequency and degree of disability. Consensus identify groups of patients in whom preventive treatment should be offered or considered, based on these parameters.99

AHS Criteria for Preventive Treatment
Preventive pharmacologic treatments should be considered for patients with migraine in any of the following situations:99
- Attacks significantly interfere with patients’ daily routines despite acute treatment
- Frequent attacks (≥ 4 MHDs)
- Contraindication to, failure, or overuse of acute treatment
- Adverse events with acute treatment
- Patient preference
AHS Recommendations for Initiating Treatment with anti-CGRP Pathway mAbs
Initial treatment prescribed by a clinician when a patient is aged ≥ 18 years and 1 of the following is met:99
4–7 monthly migraine days* and both:
- Inability to tolerate (due to side effects), or inadequate response to, 8-week trial (at a dose established to be potentially effective) of two or more prior treatment classes†
- At least moderate disability (MIDAS ≥ 11, HIT-6 > 50)
8–14 monthly migraine days* and:
- Inability to tolerate (due to side effects), or inadequate response to, 8-week trial of two or more prior treatment classes†
Chronic migraine and either:
- Inability to tolerate (due to side effects), or inadequate response to, 8-week trial of two or more prior treatment classes†
- Inability to tolerate, or inadequate response to, a minimum of two quarterly injections (6 months) of onabotulinumtoxinA
*International Classification of Headache Disorders, third edition (ICHD-3) migraine with or without aura.
† ≥ 2 of the following: (1) anticonvulsants, (2) antiepileptics (not for use in women of childbearing potential who lack an appropriate method of birth control), (3) β-blockers, (4) tricyclic antidepressants, (5) serotonin-norepinephrine reuptake inhibitors, (6) other level A or B treatment classes (established efficacy or probably effective) according to American Academy of Neurology (AAN) scheme for classification of evidence.
REFERENCES
REFERENCES
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Vuralli D, et al. J Headache Pain. 2018;19:109.
- Houtveen JH, Sorbi MJ. PLoS One. 2013;8:e72827.
- Ashkenazi A, et al. Cephalalgia. 2009;29:1042-1048.
- Main A, et al. Headache. 1997;37:492-495.
- Giffin NJ, et al. Neurology. 2003;60:935-940.
- Schulte LH, et al. J Headache Pain. 2015;16:14.
- Laurell K, et al. Cephalalgia. 2016;36:951-959.
- Lampl C, et al. J Headache Pain. 2015;16:80.
- Kelman L. Cephalalgia. 2006;26:214-220.
- Giffin NJ, et al. Neurology. 2016;87:1-5.
- Goadsby PJ, et al. Physiol Rev. 2017;97:553-622.
- Edvinsson L, et al. J Headache Pain. 2018;19:21.
- Tajti J, et al. Neuropeptides. 2015;52:19-30.
- Russo AF. Annu Rev Pharmacol Toxicol. 2015;55:533-552.
- Chong CD, et al. Neuroimage Clin. 2017;13:273-277.
- Diamond S, et al. Headache. 2007;47:355-363.
- Schreiber C, et al. Arch Intern Med. 2004;164:1769-1772.
- Lipton RB, et al. Presentation FHM1. Presented at: the American Headache Society (AHS) 61st Annual Scientific Meeting; July 11-14, 2019; Philadelphia, PA.
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Buse DC, et al. Curr Pain Headache Rep. 2012;16:237-254.
- Diamond ML. Neurology. 2002;58(9 suppl 6):S3-S9.
- Lipton RB. Headache. 2011;51:77-83.
- Hirata K. Japan Med Assoc J. 2004;47:118-123.
- Dodick DW. Semin Neurol. 2010;30:74-81.
- Baykan B, et al. Clin J Pain. 2016;32:631-635.
- Misra UK, et al. Clin J Pain. 2013;29:577-582.
- Bigal ME, et al. Neurology. 2008;70:1525-1533.
- Charles A. N Engl J Med. 2017;377:553-561.
- Charles A. Lancet Neurol. 2018;17:174-182.
- Lipton RB, et al. Neurology. 2007;68:343-349.
- Russell MB. J Headache Pain. 2005;6:441-447.
- Lipton RB, et al. Neurology. 2003;61:375-382.
- American Headache Society. Headache. 2021;61:1021-1039.
- Serrano D, et al. J Headache Pain. 2017;18:101.
- Bigal ME, Lipton RB. Curr Neurol Neurosci Rep. 2011;11:139-148.
- Bigal ME, et al. Headache. 2008;48:1157-1168.
- Manack A, et al. Neurology. 2011;76:711-718.
- Lipton RB, et al. Headache. 2016;56:1635-1648.
- Buse DC, et al. Headache. 2019;59:306-338.
- Marmura MJ, et al. Headache. 2015;55:3-20.
- Simpson DM, et al. Neurology. 2016;86:1818-1826.
- Do TP, et al. J Headache Pain. 2019;20:37.
- Silberstein SD, et al. Neurology. 2012;78:1337-1345.
- Reddy DS. Expert Rev Clin Pharmacol. 2013;6:271-288.
- Ditan [Prescribing Information]. Indianapolis, IN: Lilly USA, LLC. 2020.
- Pringsheim T, et al. Headache. 2016;56:1194-1200.
- Buse DC et al. Mayo Clin Proc. 2009;84:422-435.
- Hirsch GV, et al. Ann Neurosci Psychol. 2015;2:5.
- Yu ZB, et al. Medicine (Baltimore). 2016;95:e4824.
- Schwedt TJ, et al. Cephalalgia. 2014;34:947-958.
- Fischl B, Dale AM. Proc Nat Acad Sci U S A. 2000;97:11050-11055.
- Keller SS, Roberts N. J Anthropol Sci. 2009;87:127-151.
- Crosson B, et al. J Rehabil Res Dev. 2010;47:7-34.
- Van Dijk KRA, et al. J Neurophysiol. 2010;103:297-321.
- Xie Y, et al. Acta Pharmacol Sin. 2009;30:31-41.
- Chen Z, et al. J Headache Pain. 2017;18:112.
- Fumal A, et al. Brain. 2006;129:543-550.
- Edvinsson L, et al. Nat Rev Neurol. 2018;14:338-350.
- Walker CS, Hay DL. Br J Pharmacol. 2013;170:1293-1307.
- Hay DL, et al. Br J Pharmacol. 2018;175:3-17.
- Poyner DR, et al. Pharmacol Rev. 2002;54:233-246.
- Goadsby PJ, et al. Ann Neurol. 1990;28:183-187.
- Goadsby PJ, Edvinsson L. Ann Neurol. 1993;33:48-56.
- Lassen LH, et al. Cephalalgia. 2002;22:54-61.
- Cooray SN, et al. Mol Cell Endocrinol. 2009;300:17-24.
- Russell FA, et al. Physiol Rev. 2014;94:1099-1142.
- Lennerz JK, et al. J Comp Neurol. 2008;507:1277-1299.
- Hilairet S, et al. J Biol Chem. 2001;276:42182-42190.
- Pavlos NJ, Friedman PA. Trends Endocrinol Metab. 2017;28:213-226.
- Lane JR, et al. ACS Chem Neurosci. 2013;4:527-534.
- Bond RA, et al. Front Pharmacol. 2019;10:124.
- Raddant AC, Russo AF. Expert Rev Mol Med. 2011;13:e36.
- Edvinsson L. Br J Clin Pharmacol. 2015;80:193-199.
- Eftekhari S, Edvinsson L. Ther Adv Neurol Disord. 2010;3:369-378.
- Miller S, et al. Neuroscience. 2016;328:165-183.
- Silberstein S, et al. Headache. 2015;55:1171-1182.
- Bigal ME, et al. Headache. 2013;53:1230-1244.
- Noseda R, Burstein R. Pain. 2013;154(suppl 1):S44-S53.
- Burstein R, et al. J Neurosci. 2015;35:6619-6629.
- Hou Q, et al. Pain. 2011;152:2036-2051.
- Walker CS, et al. Ann Clin Transl Neurol. 2015;2:595-608.
- Mönnikes H, et al. Digestion. 2005;71:111-123.
- Bracq S, et al. FEBS Lett. 1994;351:63-66.
- Hay DL, et al. Pharmacol Rev. 2015;67:564-600.
- Mietlicki-Baase EG. Physiol Behav. 2016;162:130-140.
- Wimalawansa SJ. Endocr Rev. 1996;17:533-585.
- Petermann JB, et al. J Biol Chem. 1987;262:542-545.
- Eftekhari S, et al. J Pain. 2013;14:1289-1303.
- Dakhama A, et al. Curr Opin Pharmacol. 2004;4:215-220.
- Martling C, et al. Regul Pept. 1988;20:125-139.
- Al-Salam S, et al. Neuroendocrinol Lett. 2009;30:506-510.
- Cottrell GS, et al. Peptides. 2012;35:202-211.
- Hay DL, et al. Br J Pharmacol. 2018;175:3-17.
- Westermark P, et al. Biochem Biophys Res Comm. 1986;140:827-831.
- D’Este L, et al. Arch Histol Cytol. 1995;58:537-547.
- Marmura MJ, et al. Headache. 2015;55:3-20.
- Silberstein SD. Neurology. 2000;55:754-762.
- American Headache Society. Headache. 2021;61:1021-1039.
- D’Amico D, et al. Neuropsychiatr Dis Treat. 2008;4:1155-1167.
- Vecsei L, et al. Exp Opin Drug Saf. 2015 ;14:667-681.
- Ditan [Prescribing Information]. Indianapolis, IN: Lilly USA, LLC. 2020.
- Gepant [Prescribing Information]. Madison, NJ: Allergan Pharmaceuticals. 2019.
- Silberstein SD, et al. Neurology. 2012;78:1337-1345.
- Schaetz L, et al. Poster EPO3112. Presented at the 5th Congress of the European Academy of Neurology (EAN), June 29-July 2, 2019; Oslo, Norway.
- Dodick DW, et al. Headache. 2016;821-834.
- Steiner TJ, et al. J Headache Pain. 2007;8(suppl 1)S3-S47.
- Buse Buse DC et al. Mayo Clin Proc. 2009;84:422-435.
USA-334-85541